
(a) 3 CaCO 3 + 2 AlPO 4 → Ca 3(PO 4)2 + Al 2(CO 3)3 (b) Double Replacement 10. Element: Carbon Symbol: C Atomic Mass: 12.0107 Calcium carbonate reacts with aluminum phosphate to produce calcium phosphate and aluminum carbonate. Element: Aluminium Symbol: Al Atomic Mass: 26.981538 # of Atoms: 2 Mass Percent: 23.062%. Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom.
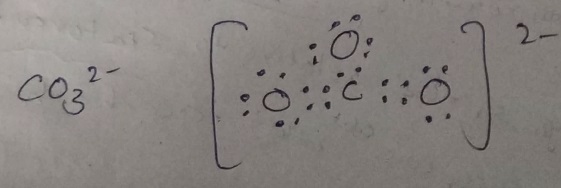
Charge of carbon in co3 plus#
Ammonium carbonate and aluminum sulfate react to yield ammonium sulfate plus aluminum carbonate. Choose the correct balanced equation for the chemical reaction described below. The number of moles of each reactant is calculated by multiplying the molarity by the volume. We make less product from the aluminum nitrate it will run out first. 0.036 moles of sodium carbonate yield 0.012 moles of aluminum carbonate. So 0.01 m,oles of sodium carbonate yield 0.005 moles of aluminum carbonate.Finally, the ammonium aluminum carbonate precursor was decomposed after calcination, thus alumina nanorods with a specific crystal form prepared. Well, if I have done my sums right, there are 14 atoms in the one formula unit of aluminum carbonate. A) hydrocalcium B) calcium dihydride C) calcium hydroxide D) calcium dihydroxide E) calcium hydride 35)The correct name of the compound Na 3 A) aluminum oxide B) dialuminum oxide C) dialuminum trioxide D) aluminum hydroxide E) aluminum trioxide 34)The correct name for CaH 2 is _. Unfortunately, statistical speaking, the values for the varying oxides and hydroxides overlap each other. A compilation of Al 2p3/2 values and modified Auger parameter values (Al 2p - KL23L23 (1D)) are presented below. Aluminum oxides and hydroxides (Al2O3, Al (OH)3 and AlOOH) are extremely difficult to differentiate by XPS. The technology of preparing of high-purity aluminium carbonate ammonium is a state of the art advanced in the world with Japanese Oaki K.K.It can be with tight and strict being controlled between 42 ± 0.5 ℃ of synthesis temperature with the fully automated control techniques.Therefore the physicals of the aluminium carbonate ammonium that obtains can satisfy the technical requirements of.Aluminum hydroxide decomposes to produce aluminum oxide and water. Calcium carbonate reacts with aluminum phosphate to produce calcium phosphate and aluminum carbonate. Because of this ability to bind phosphate in the intestine, aluminum carbonate antacids also can be used with a low. The small number after the element symbol i.Aluminum carbonate antacids can be used to treat and manage hyperphosphatemia (abnormally elevated levels of phosphate in the blood) since they bind phosphate in the intestine and prevent it from being absorbed into the body. To find the total number of atoms in Al2(CO3)3 (Aluminum carbonate) we’ll add up the number of each type of atom.


 0 kommentar(er)
0 kommentar(er)
